How to Select Power Supplies for Medical Devices

Ensuring that electrical equipment is safe for use is important in any application, but in a medical context, it can be particularly challenging. In this context, safeguarding extends beyond the equipment operator. Medical devices such as ultrasound probes, blood pressure monitors, and oximeters are often used close to patients or might have connections to them. This raises the potential for the patient to receive an electric shock, which could have serious consequences. To mitigate such risk, both the power supply and the medical equipment itself need to meet strict safety criteria.
This article aims to assist in selecting the correct power supply for medical applications. It explains the main factors to consider in identifying the optimal power supply option and provides a brief overview of some of the terminology involved before demonstrating how to apply assessment criteria in practice.
What is a Medical-Rated AC-DC Power Supply?
AC-DC power supplies for medical equipment must be evaluated by the equipment manufacturer to determine whether they require certification to the latest medical standards. The type of power supply is generally determined by the application, with the critical determining factor being whether the patient will be in direct contact with the equipment.
IEC/EN 62368 covers the electrical safety of mains-powered equipment such as computers and audio/video systems. The standard applies to the power supply, any subsystems, and the end system. A power supply that conforms to IEC/EN 62368 but is not medically approved may be suitable for some healthcare applications.
IEC 60601-1 (now in its 4th edition) defines the safety criteria and specifications for any equipment connected to a mains power supply that is used to monitor, diagnose, and treat a patient. Such equipment includes heart rate monitors, drug dispensers, and surgical lasers. This standard applies to the complete system, so external power supplies and wall-mounted power supply adapters need to be certified as well.
It should be noted that a power supply that states it is certified to medical approval standards does not always mean that the end product it is powering will conform to IEC/EN 60601.
Compared with general-purpose power supplies, medical-rated versions characteristically have very low leakage current between the live and isolated sides and have a larger creepage distance across the isolation barrier, among other internal requirements. To clarify, creepage is the distance measured along the surface of insulating material between two points, and clearance is the direct distance through air between them.
Now we have a top-level definition of a medical-rated power supply, let’s look deeper into the selection process and the terminology involved.
Means Of Protection
IEC 60601-1 has specific requirements for “Means Of Protection” (MOP) and these cover the operator (MOOP) as well as the patient (MOPP).
Essentially, a MOP (Means of Protection) is a means of preventing a patient or an equipment operator coming into contact with the lethal mains voltage of the medical device. Examples include the use of safety insulation or a protective earth connection. In addition, IEC 60601-1 defines the minimum distances for creepage, air gap clearances, and other protective impedances, all of which can be used in combination to achieve compliance.

Figure 1 - Insulation Diagram of a Murata Power Solutions (MPS) medical-rated power supply
Figure 1 illustrates the areas within a medical-rated power supply where MOPs (Means of Protections) are applied. This example includes different protection levels (and types) where:
- Area “A” = 1 x MOOP
- Area “B” = 1 x MOPP
- Area “C” = 2 x MOPP
- Area “D” = 1 x MOPP
- Area “E” = 1 x MOPP
- Area “F” = 1 x MOPP
This can be important in the end (system) application. If the power supply output is connected to the patient without 1 x MOPP isolation from the power supply, there is a potential system-level non-compliance. Medically-approved power supplies, such as the PQU650 series of open-frame, 650 W-rated AC-DC power supplies from Murata, have been assessed for safety by a third party accreditation agency, so the end user can be confident that adequate MOP (Means of Protection) are in place.
However, there is still a requirement to determine which level of MOP (Means of Protection) is applicable to the intended deployment. When only operators will come into contact with the equipment, 2 x MOOP is sufficient. When patients could come into contact, even accidentally, then 2 x MOPP must be applied.
Figure 2 (extracted from IEC 60601-1 Edition 3) shows the decision-making process, in flowchart form, which manufacturers should use to assess and determine the type of MOP (Means of Protection) required for any medical equipment application:

Figure 2 - MOP (Means of Protection) Decision Tree
The highest level of safety protection is achieved by using 2 x MOPP. Power supply manufacturers often highlight whether their power supplies meet medical approvals, specifying whether the unit has 1 MOPP or 2 MOPP. Buyers should verify their requirements and review the proposed power supply datasheet before placing an order.
An AC/DC power supply that provides only 1 x MOPP can still be integrated into a design requiring 2 x MOPP (or MOOP) by adding an isolated DC/DC converter. While this incurs additional costs, it might be the preferred solution, especially if more than one DC rail is needed.
In certain cases, choosing a single 2 x MOPP power supply that is compatible with all medical products could be advantageous. The price premium for such a supply, compared to an IEC/EN 62368 unit, is likely to be minimal and may be compensated by the savings in reduced inventory complexity and sourcing costs.
What is an Applied Part?
As Figure 2 illustrates, the primary decision to be made is whether the equipment in question is an Applied Part, so it is worthwhile to examine this term more closely. An Applied Part is one that can be in contact with, or applied to, the patient. There are three types of Applied Parts to consider for each intended application:
- Type B (Body): this type is (or may be) in contact with the patient’s body, which may be connected to earth. The associated risk is excessive patient leakage current. Examples of equipment that may be Type B rated are LED lighting, medical lasers, MRI/CT body scanners, hospital beds, and phototherapy equipment.
- Type BF (Body Floating): this type is also in contact with the patient’s body, but the patient is not directly connected to earth (i.e. they are considered to be “floating” with respect to earth). Again, the risk is excessive patient leakage current. Examples of this type of equipment are blood pressure monitors, incubators, and ultrasound equipment.
- Type CF (Cardiac Floating): this type categorises equipment for direct cardiac (or bloodstream) application. As such, the patient leakage current maximum limit allowed is extremely low. Examples include dialysis and surgical equipment.
If none of these categories apply, then the intended application does not (mandatorily) require an Applied Part rated power supply, although this does not necessarily mean that some form of medical certification will not be required.
Once the type of Applied Part has been determined, research of suitable products can be undertaken to ensure that they not only meet the operational requirements for the intended application, but also the correct level of MOP (Means of Protection).
Leakage Currents and their Influence on Power Supply Selection
Leakage currents are defined as unwanted current, which can be AC or DC in nature or a combination of both, flowing from the input (AC source side) and/or the output side of the power supply.
Leakage currents are an important factor in determining the correct level of MOP (Means of Protection), but they can be a source of confusion. IEC 60601-1 defines three main types of leakage current, which are summarised below. (There is a fourth leakage current mentioned in IEC 60601, however this is out of the scope of this article).
- Earth Leakage Current
Earth leakage refers to the electrical current in a system that finds an alternative return path other than the active and neutral conductors. It is associated with a residual current of small magnitude, typically measured in milliamperes (mA).
Figure 3 illustrates the current flowing from the AC input source (usually via capacitance within the power supply) through the earth conductor (Protective Earth). This capacitance can be parasitic (stray) or intentionally placed between the AC source connections (live and neutral) and earth to reduce EMI emissions.
If the earth connection is secure, there is no risk to either patient or operator, as no current can flow through them to earth, even when in direct contact with the enclosure. Under normal conditions, the allowable current is 5mA, and for Single Fault Conditions (SFC), it can be up to 10mA.

Figure 3 - Earth Leakage Current Path
2. Enclosure (Touch) Leakage Current
Enclosure leakage current is defined as the current that flows from an exposed conductive part of the enclosure to earth through a conductor other than the protective earth conductor.
Figure 4 shows the current that would flow if the patient (or the operator) were connected to earth and in direct contact with the enclosure. Under normal conditions, the allowable current is low (100μA) and for Single Fault Conditions (SFC) is allowed to be 500μA.

Figure 4 - Enclosure (Touch) Current Path
3. Patient Leakage Current
Patient leakage current is the current that flows through a patient connected to an applied part or parts. It can either flow from the applied parts via the patient to earth or from an external source via the patient and the applied parts to earth.
Figure 5 shows the current flowing between an item of medical equipment and through the patient to earth. The allowable current is 100μA. Under Single Fault Conditions (SFC) this allowable current is 500μA.

Figure 5 - Patient Leakage Current Path
Leakage current can also flow from another source i.e. another item of medical equipment connected to the patient and to earth (see Figure 6).

Figure 6 - Patient Leakage Current Path (External Source)
Staying Grounded
Having determined the type of leakage path that requires mitigation, it is time to determine the most appropriate power supply. There are two classifications of equipment given in IEC 60601-1(Edition 3) that will help to determine the level of protection your medical equipment offers against electric shock.
Class I equipment is intended to be provided with a Protective Earth (PE). The insulation system between the primary and all metal, or internal metal parts, is basic. In other words, a PE connection must be present on the metal enclosure of the equipment. Equipment intended for Class I connection to the utility is provided with three connector terminals (one of which will be designated the PE contact). Equipment intended for Class I connection to the utility are provided with a connector that will be provided with a PE contact. (An example can be seen in Figure 7).

Figure 7 - A Murata PQU650M-xxP Three Pin Header With Integral PE For Class I Implementation
Class II equipment is not intended to be provided with a PE connection. As a consequence, the level of insulation between the primary and all metal or internal metal parts is required to be supplemented by double or reinforced insulation. Equipment intended for Class II connection to the utility are usually provided with a two-terminal connector (see Figure 8 for an example).

Figure 8 - A Murata PQC250 Series Two Pin Header With Separate PE Terminal For Class II Implementation
Usually, the enclosure of the equipment is non-conductive (plastic). However, metal cases are allowed for Class II products assuming that 2 x MOOP is provided from primary to PE/Ground and the End User. Crucially, it is the responsibility of the End User to determine which insulation Class is required. This can be determined by asking two simple questions:
- Is the enclosure intended to be conductive (metal)?
- If so, is a PE connection available?
The answers will enable a power supply with a suitable connector to be identified.
Power supply selection in practice
Now we understand the characteristics of a medical-grade power supply, we can put our knowledge into practice. In this case, we will consider the power supply requirements for a hospital bed.
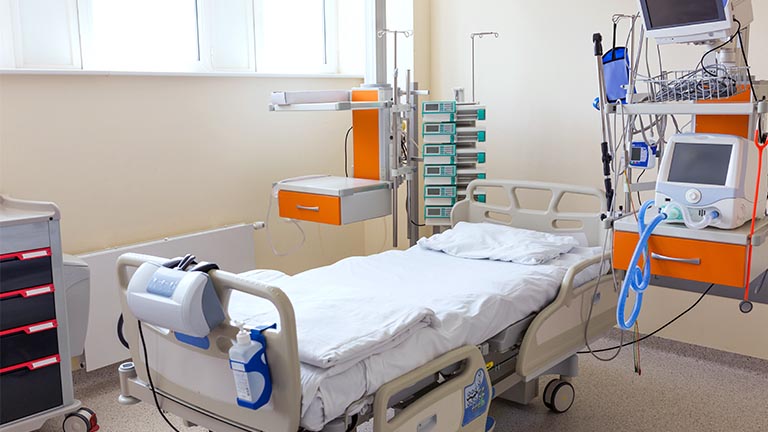
Figure 9 - What Power Supply Criteria Applies To The Modern Hospital Bed?
Modern hospital beds are complex mechanical devices that use various motors and hand controls to operate them. From an electrical safety perspective, there are two potential ways to power a bed. It could incorporate a built-in AC power supply or use an external low voltage power supply. The former approach is most common since it keeps the number of external cables to a minimum.
Instead of using a mains power supply, there are benefits to using an external low voltage supply. Safety concerns will be lower if the bed is connected to fewer electronics, and with a supply under 24 Volts annual portable appliance testing is not required. However, an external power supply will need to be mechanically robust, have an ingress protection rating of IP4X or above, and the connector cables must have adequate strain relief.
There is a high degree of risk that the patient could come into contact with conducting parts due to the metal bed frame, so a 2 x MOPP power supply is required to meet the safety requirement. Such a power supply will use a Class II protective earth, and since it has an external low voltage output it will not require annual PAT testing, making it popular for hospital bed applications.
Other factors to consider include the power supply shape, weight, and the vulnerability of damage or fluid ingress. Also, noise is of particular concern for many hospital applications, so the availability of a power supplies without fans are essential. A range of external convection-cooled 2 x MOPP power supplies with outputs up to 220 watts are available from a variety of reputable manufacturers.
In Summary
There are multiple criteria to consider when selecting a suitable power supply for medical applications. While this article is not exhaustive, it provides an overview of some common questions encountered when initially choosing a suitable power supply for medical devices. We have discussed the importance of the MOP (Means of Protection) for both the Patient and Operator in power supply selection. We have learned how to determine whether medical equipment is an “Applied Part” and have reviewed the different types of leakage current, gaining an understanding of grounding in relation to power supply selection. Finally, we have illustrated how this knowledge can be practically applied, using the example of a modern hospital bed.
This article also includes examples of Murata Power Solutions, suitable for a wide range of medical equipment applications. Our team of experts is available to provide guidance and assistance in selecting a recommended model for medical equipment deployment and to offer technical support.
Avnet Abacus' industry-leading linecard features products from the world's top power suppliers, designed and manufactured to medical safety standards for various applications:
● Medical imaging: ultrasound scanners, MRI, CT, PET, X-Ray
● Surgical devices: robotics, electrosurgery, laser surgery
● Medical devices: patient therapy, patient monitoring, patient transport, beds, ventilators, powered air-purifying respirators, anaesthesia machines, aspiration and suction pumps, autoclaves, sterilisers, blood chemistry analyzers, centrifuges, and more
● Dental equipment: oral care systems, CAD/CAM systems, digital radiography
● Wellness and beauty: laser hair removal, UV light therapy, laser therapy, ultrasound
For further information: https://my.avnet.com/abacus/products/product-highlights/murata-power-supplies-for-medical-devices/


Ask an expert
Have a question? Our regional technical specialists are on hand to help.



