The IoT is ready for the era of virtual clinical trials

The IoT is already being used to share medical data; soon it will be home to virtual clinical trials.
The repercussions of a global pandemic like COVID-19 are felt long after the initial impact. The immediate effects of restricted movement and physical interactions are felt strongly in everyday activities such as going to work and leisure activities. Many things we do in person on a day-to-day basis can be carried out online, thanks to the internet. One area where a physical presence is essential, however, is clinical trials. During a pandemic, clinical trials are arguably even more essential, but the limitations imposed on travel impede progress.
Continuum Clinical, a specialist in global clinical trial enrollment, reported that most of its clients had to reschedule clinical trial studies during the height of the pandemic because of the restricted access to participants. The culmination of self-isolation, reduced access to work sites and general restrictions on travel caused pharmaceutical companies and clinical research organizations (CROs) to rethink how they could carry out trials.
With the Internet of Things (IoT) stepping in to fill the void in other areas, clinicians asked if the IoT could also be used to carry out clinical trials in a virtual world. Could using the latest technology to monitor patients in their own homes be the solution? The short answer is “yes,” and the longer response is that the IoT will be instrumental in the way future clinical trials are conducted. Moving to virtual trials could also reduce the time taken to conduct clinical trials from years to months, or in some cases even less than a day.
How clinical trials are conducted today
Clinical trials have always been an important part of drug development. Studies provide evidence of how efficient a drug can be, but getting that data takes time. This makes the process long and expensive. It isn’t uncommon for clinical trials to last years and the cost to reach several billion U.S. dollars.
The breakdown of a typical trial comprises four phases, as shown below. Each phase has a specific purpose. The associated time and cost of each phase will depend on the drug being developed. Each phase targets a different group, so participant recruitment is needed for each stage. Even during times of no restrictions on movement or travel, this introduces its own challenges.
| Phase 1 |
The drug is tested on a small group of healthy people. This provides indication of harmful side effects. |
| |
|
| Phase 2 |
The drug is tested on a group of infected patients. This provides evidence of its efficacy. |
| |
|
| Phase 3 |
The group is increased, testing more affected patients to assess effectiveness of the drug being developed in the longer term. |
| |
|
| Phase 4 |
After gaining Food and Drug Administration (FDA) approval, the drug will be tested for a longer period across a wider and more diverse group of patients. |
In the future, the IoT will change this and provide easier access to clinical trial participants. This will alleviate the very real issue of finding patients for trials during each phase of drug development.
Although drug development is expensive, this isn’t only about cost. Finding and retaining participants, at any cost, is difficult. Drug companies tend to be large and are therefore located in or close to urbanized areas. This limits the catchment area for participants, with those not close to the facility being much less likely to participate in clinical trials.
Research carried out in 2018 by the Center for Information and Study on Clinical Research Participation (CISCRP) revealed that 80% of clinical trials are put off due to enrollment issues, and 87% of trials fail to recruit enough patients. The same study indicated that 54% of potential patients would be willing to participate in virtual trials.
These figures and corresponding evidence prompted the FDA to issue draft guidance in 2019 urging improvement in the diversity of participants in clinical trials. The section titled “Make Trial Participation Less Burdensome for Participants” indicates that advanced mobile tools should be implemented to limit the need for site visits by patients.
Using the IoT for clinical trials
Cost and access to patients are two important aspects of clinical trials, but there are others. These include:
- The need for data entry, which is often carried out manually
- The accuracy of vital measurements
- Access to well trained and experienced staff to conduct the trials
- The availability and access to data
These are predominantly data-centric considerations. The IoT was effectively built to capture real-world data and handle complex data sets. Also, the IoT doesn’t know what is real and what is virtual, so it has no preconceptions of the different domains. A virtual trial could be as simple as capturing data from a patient in a facility, to monitoring vital statistics halfway across the world.
Data can be collected by a trained member of staff or a wearable sensor. The smart connected device will capture patient data and send it securely to the CRO. This is already happening using devices as common and ubiquitous as smartphones and smartwatches. The use of home-based medical devices connected to the internet is accelerating. Patients already monitor their own medical conditions, including blood sugar levels, heart health, weight, sleep patterns and much more. This data is shared with medical professionals who may be many miles away. The same technology is applicable to virtual clinical trials, making it easier to conduct trials for CROs and participants.
The advantages for CROs include:
- Patients can use their own smart devices.
- Participants and patients are monitored remotely.
- The IoT provides instant and continuous access to live data.
- Smart triggers and alerts can be used to provide faster response.
- A secure IoT infrastructure supports remote screening, patient verification and online recruitment.
- Drugs and other supplies can be delivered to the patient’s home.
- Participants feel more connected to the trial through their always-on connected devices.
As the concept takes hold, the data captured remotely can be preconditioned for advanced technologies such as artificial intelligence (AI). This will enable medical experts to leverage technology, data and their preferred analytics in a more integrated way to support risk-based monitoring (RBM). It is important to adopt RBM for clinical trials, as it identifies risks that could affect the quality of any data gathered. An RBM platform can provide the following features:
- Improve the quality of a study
- Provide greater patient safety
- Deliver better monitoring
- Provide centralized access to data
- Add transparency to the trial
- Help with implementing corrective actions
In the near future, participants and patients will expect a CRO to offer the option of a virtual clinical trial. This is part of the patient-central approach that CROs are being encouraged to adopt. But this isn’t only about the patients; the CROs will benefit from virtual clinical trials too, as shown below.
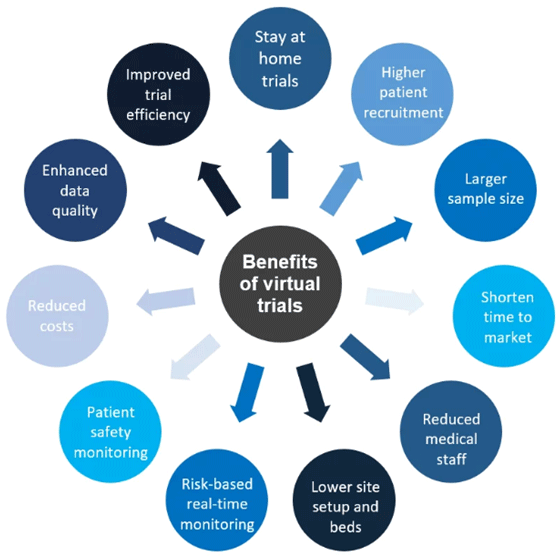
CROs are using the IoT to carry out virtual clinical trials, which brings benefits to the CRO and to the participants.
Going virtual for clinical trials
The IoT will directly benefit patients and CROs. Virtual clinical trials will become the norm, not the exception. This will happen because there is a real, demonstrable need for it to happen. Technology can sometimes feel imposing, but in this case, it will be empowering.
There will, of course, be a learning curve for everyone involved. Just like today, each clinical trial will have common requirements, but they will also be individual and unique. This means customizing the virtual trial for every instance. Fortunately, working in a virtual domain supports easy customization.
If you would like to find out how our team of experienced professional IoT developers can help your team of experienced professional clinicians, please get in touch.
About Author
Philip Ling
Philip Ling is a senior technology writer with Avnet. He holds a post-graduate diploma in Advanced M...
msembedded content library/articles/iot-ready-for-virtual-clinical-trials
The IoT is ready for the era of virtual clinical trials


